Herranz Group
The research interests of Herranz Group is analysis of gene functions in development and disease, using Drosophila.
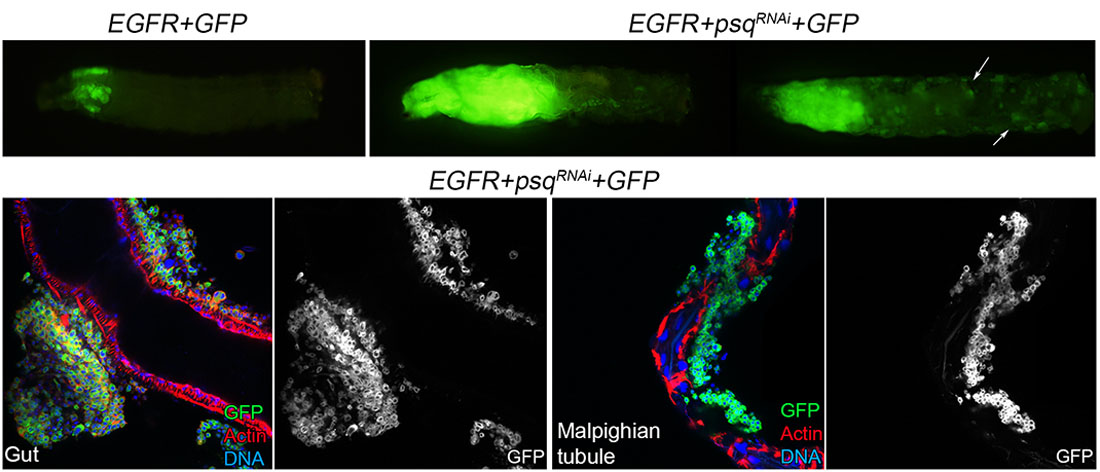
- understand how different mutations cooperate during the process of cellular transformation
- study the oncogenic potential of epithelial tetraploid cells that result from cytokinesis failure
- better understand how the metabolic changes taking place in cancer cells contribute to cellular transformation and metastasis
- determine the role that miRNAs play in growth control during normal development and cancer
Cancer develops in a complex mutational landscape. Cancer cells accumulate ‘driver mutations’ that are causally linked to disease, and ‘passenger mutations’ that, although present, have limited impact on disease. In recent years, factors encoded by cancer genes have become targets for successful anticancer drug development. Although cancer genome sequence data provide an unparalleled depth of information about the mutations present in different cancer genomes, identification of genetic alterations contributing to tumorigenesis and metastasis calls for the use of simple genetic model systems.
We have engineered Drosophila strains that activate different cancer-driver mutations. These flies allow, with a single genetic cross, the introduction of new mutations to identify genes that, when combined with driver mutations, lead to tumor formation and metastasis.
- Genetic models of oncogene cooperation
- microRNAs in growth control, development, and cancer
- Cytokinesis failure and cancer
- Cancer metabolism
- dTcf/Pangolin suppresses growth and tumor formation in Drosophila. Song S, Andrejeva D, Freitas FCP, Cohen SM, Herranz H. Proc Natl Acad Sci USA. 2019 Jun 24. pii: 201816981. doi: 10.1073/pnas.1816981116.
- Eichenlaub T, Villadsen R, Freitas F, Andrejeva D, Aldana BI, Nguyen HT, Petersen OW, Gorodkin J, Herranz H*, and Cohen SM*. Warburg effect metabolism drives neoplasia in a Drosophila genetic model of epithelial cancer. Curr Biology. 2018 Oct 22;28(20):3220-3228.e6. doi: 10.1016/j.cub.2018.08.035. Epub 2018 Oct 4. *Corresponding author.
- Gerlach SU, Eichenlaub T, and Herranz H. Yorkie and JNK Control Tumorigenesis in Drosophila Cells with Cytokinesis Failure. Cell Reports. 2018 May 1;23(5):1491-1503. doi: 10.1016/j.celrep.2018.04.006.
- Eichenlaub T, Cohen SM*, and Herranz H*. Cell competition drives the formation of metastatic tumors In a Drosophila model of epithelial tumor formation. Current Biology. 26, 1–9 February 22, 2016. *Corresponding author.
- Herranz H*, Weng R, and Cohen SM*. Crosstalk between Epithelial and Mesenchymal Tissues in Tumorigenesis and Imaginal Disc. Current Biology. 24, 1–9, July 7, 2014. *Corresponding author
- Herranz H*, Hong X*, Hung NT, Voorhoeve PM, and Cohen SM. Oncogenic cooperation between SOCS family proteins and EGFR identified using a Drosophila epithelial transformation model. Genes and Development. 2012 Jul 15; 26: 1602-1611. *These authors contributed equally to this work
 Group Leader
Group Leader
Héctor Herranz Muñoz
Associate professor
hherranz@sund.ku.dk
(+45) 35 32 73 98 / (+45) 30 45 00 26
CV, publications, etc.


