Schjoldager Group
The main goal of our research is to discover and dissect molecular protein functions that impact human physiology and common diseases and dispositions.
The main goal of our research is to discover and dissect molecular protein functions that impact human physiology and common diseases and dispositions. My group focuses on post translational modifications and in particular protein glycosylation, merging protein biology, glycoscience, and biomedicine within classical cell biology. We use state-of-the-art gene engineering technologies, mass spectrometry and biophysical analysis in combination with animal and cell models to explore specific functional and structural impact of protein glycosylation as well as disease-causing driver functions of the enzymes controlling protein glycosylation. The three major research themes in my lab can be found under "Current project areas".
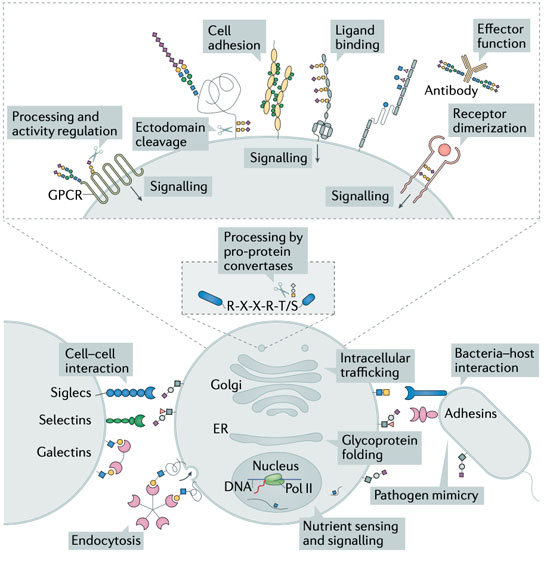
1: Biological Functions of Glycans - Glycosylation serves to tune and diversify functions of proteins Schjoldager et al., 2020. Several illustrative examples with clear impact on disease, document the importance of different types of glycosylation in regulating protein functions, however, we are still at a stage of infancy in recognizing and appreciating the role of glycosylation in common diseases. My group has lead the mapping of the mammalian O- glycoproteome in tissues and biofluids and discovered O-glycans on important classes of proteins with functional roles Bennett et al., 2012; Schjoldager et al., 2010, 2012, 2015; Steentoft et al., 2013; Goth et al., 2015, 2017, 2018; King et al., 2018; Khetarpal et al., 2016; Hansen et al., 2017; Wang et al., 2018, Tian et al., 2019; Hansen et al., 2019; Madsen et al. 2020). At the specific glycosites, glyco structures (core-structures and capping) can determine the ultimate outcome and regulatory potential of glycosylation and there is abundant evidence that the structure of glycans on proteins regulates diverse protein interactions and biological functions. In this line, through genetic engineering in cell lines, we can produce glycoproteins with specific glycosylation sites and defined glycan structures for biophysical and biochemical analysis as well as application in biological models. In this project line we are following the role of specific glycoproteins to discover other fundamental functions of O-glycosylation.
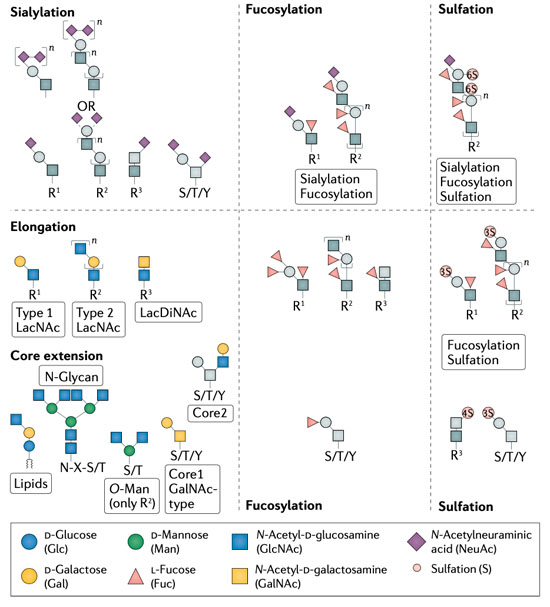
2: Protein-protein interactions in the context of complex carbohydrates – This project line aims to analyse and characterise the molecular mechanisms underlying glycoprotein functions. Protein-protein interactions represent a fundamental aspect of cell biology and great efforts are devoted to exploring and defining the protein interactome. Common for most current analytic strategies is that these ignore prevalent PTMs like glycosylation that affect protein biophysical properties and structure and serve essential roles in fine-tuning protein functions (Schjoldager et al., 2020). We have spearheaded the mapping of the mammalian glycoproteome in tissues and biofluids and discovered that O-glycosylation is a major PTM found on important classes of proteins both soluble and embedded in the plasma membrane and glycocalyx (Goth et al., 2018; Hansen et al., 2017; Khetarpal et al., 2016; King et al., 2017; Madsen et al., 2020). Protein glycosylation is well-documented to be important for the single cell and the complex organism and when glycosylation goes awry, disease develops as described above. Depending on how affected the glycosylation apparatus is, subtle phenotypes as exemplified by familial hyperphosphatemia calcinosis with loss of GALNT3 may develop, but when protein glycosylation is more broadly impaired severe diseases develop, most with clear metabolic and neurological involvement, and total loss of protein glycosylation is incompatible with life. Progress in discerning aetiology and approaching molecular mechanisms involving glycoproteins in neuronal development and metabolic signalling has been halted by technical limitations in studying protein glycosylation. In this project line we are exploring O-glycosylation sites in neuronal and neuroendocrine cells and tissues as a discovery platform for novel protein functions, protein-protein interactions, and rational design of protein therapeutics for a range of common diseases.

3: Regulation of protein O-glycosylation and genetic deficiencies- Mutations leading to structural defects in glycosyltransferase genes and severe multisystemic diseases as described above are extremely rare (Hansen et al., 2014; Zilmer et al., 2020). In contrast, genome-wide association studies (GWAS) quite frequently find that single nucleotide polymorphisms (SNPs) in glycosyltransferase genes, and in particular GALNT genes, associate with complex traits or common diseases/conditions like kidney disease (GALNT11), osteoporosis (GALNT3), dyslipidemia (GALNT2) and body mass index (GALNT10) (Joshi et al., 2018). To validate these candidate genes the task is to identify specific roles of individual GALNTs to uncover disease-causing mechanisms and explore diagnostic and therapeutic options. This task may be guided by phenotypic characteristics associated with complete deficiencies of GALNT genes in animal models and rare patients, as well as from GWAS phenotypic traits. We are using such guidance and provided the first molecular dissections of highly specific functions of GALNTs that cause human diseases (Khetarpal et al., 2016; Wang et al., 2018, Tian et al., 2019, Zilmer et al., 2020) supporting the hypothesis that dysregulation of GALNTs underlie a range of common complex diseases through improper fine-tuning of protein functions. To explore this concept further, we are developing glycoengineered cell and animal models that will provide us with much needed basic insight into how glycosyltransferases are regulated at the transcript and enzyme activity level and accordingly expand the workspace for discovery and design of selective inhibitors of individual GALNT enzymes needed for in vivo studies.
- Schjoldager KT, Narimatsu Y, Joshi HJ, Clausen H. Global view of human protein glycosylation pathways and functions. Nat Rev Mol Cell Biol (12):729-749; 2020.
- Madsen TD, Hansen LH, Hintze J, Ye Z, Jebari S, Andersen DB, Joshi HJ, Ju T, Goetze JP, Martin C, Rosenkilde MM, Holst JJ, Kuhre RE, Goth CK, Vakhrushev SY, Schjoldager KT*. An atlas of O-linked glycosylation on peptide hormones reveals diverse biological roles. Nat Commun 11(1):4033; 2020.
- Zilmer M, Edmondson AC, Khetarpal SA, Alesi V, Zaki MS, Rostasy K, Madsen CG, Lepri FR, Sinibaldi L, Cusmai R, Novelli A, Issa MY, Fenger CD, Abou Jamra R, Reutter H, Briuglia S, Agolini E, Hansen L, Petäjä-Repo UE, Hintze J, Raymond KM, Liedtke K, Stanley V, Musaev D, Gleeson JG, Vitali C, O'Brien WT, Gardella E, Rubboli G, Rader DJ, Schjoldager KT*, Møller RS. Novel congenital disorder of O-linked glycosylation caused by GALNT2 loss of function. Brain 143(4):1114-1126; 2020.
- Tian E, Wang S, Zhang L, Zhang Y, Malicdan MC, Mao Y, Christoffersen C, Tabak LA, Schjoldager KT*, Ten Hagen KG. Galnt11 regulates kidney function by glycosylating the endocytosis receptor megalin to modulate ligand binding. Proc Natl Acad Sci USA 116(50):25196-25202; 2019.
- Narimatsu Y, Joshi HJ, Nason R, Van Coillie J, Karlsson R, Sun L, Ye Z, Chen YH, Schjoldager KT, Steentoft C, Furukawa S, Bensing BA, Sullam PM, Thompson AJ, Paulson JC, Büll C, Adema GJ, Mandel U, Hansen L, Bennett EP, Varki A, Vakhrushev SY, Yang Z, Clausen H. An Atlas of Human Glycosylation Pathways Enables Display of the Human Glycome by Gene Engineered Cells. Mol Cell 75(2):394-407.e5; 2019. I
- Hansen LH, Madsen TD, Goth CK, Clausen H, Chen Y, Dzhoyashvili N, Iyer SR, Sangaralingham SJ, Burnett JC Jr, Rehfeld JF, Vakhrushev SY, Schjoldager KT*, Goetze JP. Discovery of O-glycans on atrial natriuretic peptide (ANP) that affect both its proteolytic degradation and potency at its cognate receptor. J Biol Chem 294(34):12567-12578; 2019.
 Group Leader
Group Leader
Katrine Ter-Borch Gram Schjoldager
Associate Professor
PhD-coordinator at ICMM
schjoldager@sund.ku.dk
(+45) 35 33 55 41
CV, publications, etc.



