Larsen Group
We are interested in understanding the fundamental aspects of human embryonic development and birth defects. We have special focus on the genetic and functional aspects of human cardiac development and congenital heart disease, but we are also interested in investigating genetic and molecular mechanisms involved in other areas of human embryonic development, including development of the brain.
We are interested in understanding the fundamental aspects of human embryonic development and birth defects. We have special focus on the genetic and functional aspects of human cardiac development and congenital heart disease, but we are also interested in investigating genetic and molecular mechanisms involved in other areas of human embryonic development, including development of the brain.
Congenital heart disease (CHD) is gross structural abnormalities of the heart and intrathoratic vessels. This is the most common group of inborn malformations, with an incidence of almost 1% in the population.
Ninety percent of CHD patients survive to adulthood and an estimated 12 million people are living with CHD worldwide. In many cases, CHD can be regarded as a life-long chronic disorder; adults with CHD have increased mortality, patients suffer from severe comorbidities, in particular neurodevelopmental disability, and experience a progressive decline in cardiopulmonary function, which may lead to heart failure.
The etiology of CHD is complex but with a significant genetic component. Large families with CHD are very rare and CHD is a very heterogeneous disorder, thus it is difficult to identify the causative variants in CHD patients and the genetics and pathophysiology of CHD is generally not well understood.
Increased knowledge of disease genes, variants and pathophysiological mechanisms associated with cardiac development and CHD is necessary for improving prevention of CHD as well as early diagnosis, prediction and support of CHD patients suffering from co-morbidities.
This research may also provide a basis for development of new drug therapies e.g. for minor cardiac defects like small atrial- and ventricular defects or prevention, early diagnosis or treatment of late-onset aortic valve disease. Furthermore, identifying and understanding molecular mechanisms in human cardiac development, especially the subset of genes and molecular networks, which regulate cardiomyogenesis is likely to have an impact on the development of therapeutic means for regeneration of cardiomyocytes in myocardial infarction and congestive heart failure.
Heart development is a complex process, which involves establishment of a primitive heart tube, looping of the heart tube, followed by chamber formation, chamber septation and development of the cardiac valves and outflow tract (figure 1).
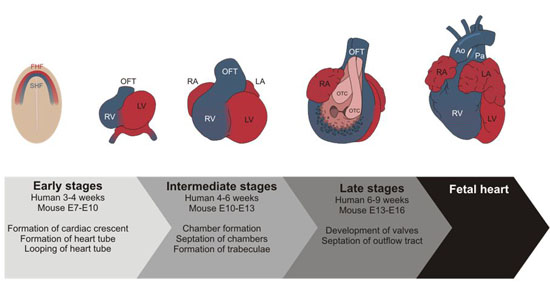
Figure 1. Developmental stages of the heart (Koefoed et al., 2014).
Development of the heart is coordinated by a significant number of cellular signaling networks like the Hedgehog, WNT, TGF-beta signaling pathways. Furthermore, many known human CHD disease genes are involved in different aspects of cellular signaling (figure 2). Thus, we are also interested in exploring the mechanisms and organelles which regulate signaling transduction during heart development and cardiomyogenesis, for example the primary cilium
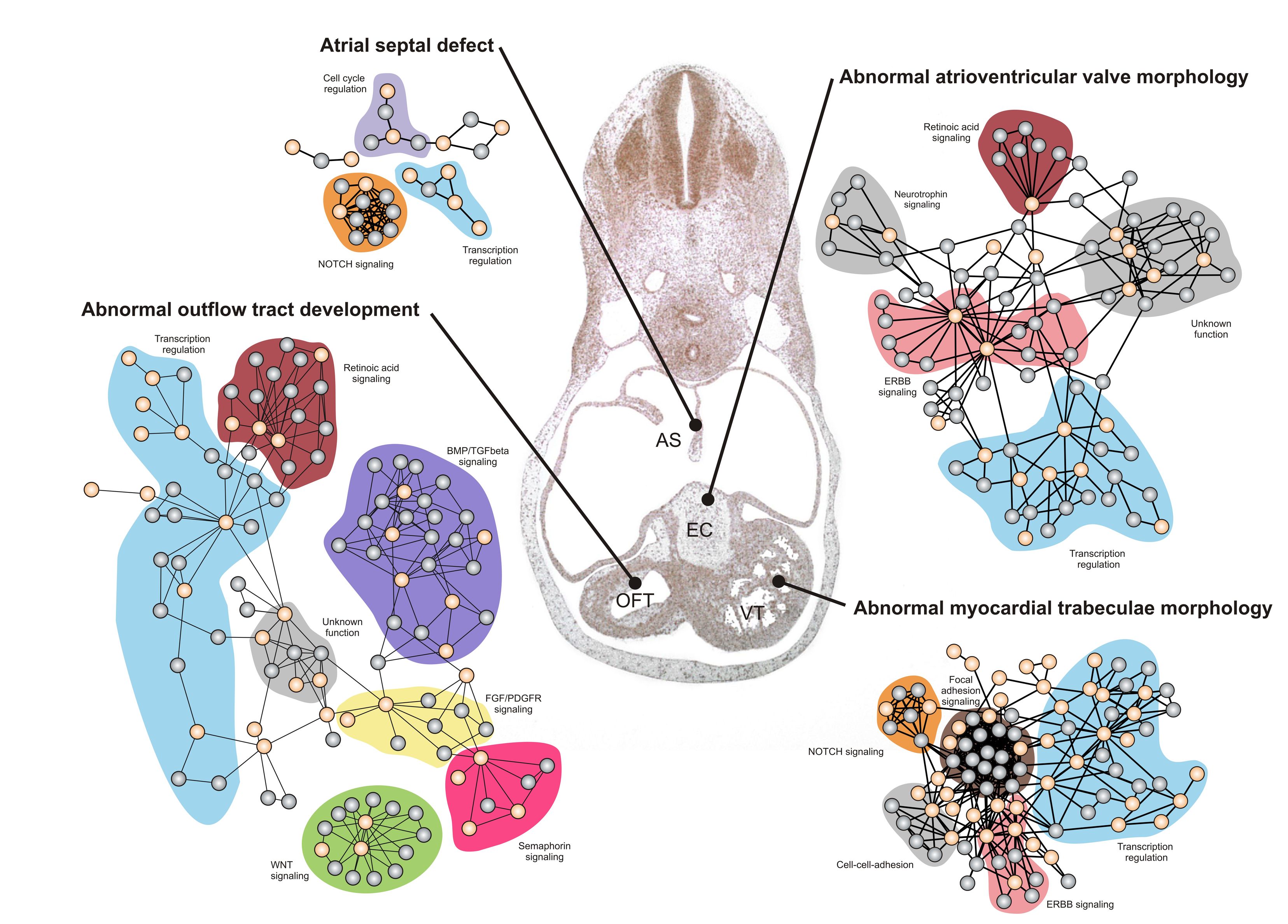
Figure 2. Developmental networks involved in development of specific cardiac structures (Lage et al., 2010).
We explore different genomic strategies for identification of genes, genomic regulatory regions and molecular networks involved in embryonic development and disease. To this end, we perform genetic analysis of patients and investigate the function of the candidate genes or signaling pathways by expression analysis in embryonic tissues and functional assays in cell models and zebrafish.
In collaboration with Prof. Vibeke Hjortdal and Prof. Henning Bundgaard, Copenhagen University Hospital, we investigate genetic and functional aspects of congenital heart disease.
In collaboration with the Cilia Group at Department of Biology, UCPH we are investigating how the primary cilium is coordinating signaling pathways during embryonic development.
In collaboration with Dr. Karen Grønskov, Copenhagen University Hospital, we investigate genetic and functional aspects of human eye development and disease.
-
Thomsen OK, Fialová JL, Doganli C, Herrera-Cid C, Møllgård K, Benmerah A, Larsen LA, Christensen ST (2025) Primary cilia as architects of the neocortex: roles in brain development, function, and microcephaly. Dev Cell 60:3364-3386.
- Doganli C, Baird DA, Ali Y, Thomsen OK, Audain E, Jessen L, Truelsen PM, Mogensen JB, Holm MS, Apolínová K, Buttò L, Diamanti M, Fialová JL, Wade EM, Robertson SP, Pedersen LB, Argiro L, Lescroart F, Hitz M-P, Christensen ST, Larsen LA. TAK1 operates at the primary cilium in non-canonical TGFB/BMP signaling to control heart development, 2024, BioRxiv 592628.
- Audain E, Wilsdon A, Dombrowsky G, Sifrim A, Breckpot J, Perez-Riverol Y, Loughna S, Daly A, Antoniou P, Hofmann P, Perez-Riverol A, Kahlert A-K, Bauer U, Pickardt T, Klaassen S, Berger F, Daehnert I, Dittrich S, Stiller B, Abdul-Khaliq H, Bu'lock F, Uebing A, Kramer H-H, Iyer V, Larsen LA, Brook JD, Hitz M-P: Assessing the contribution of rare variants to congenital heart disease through a large-scale case-control exome study, 2023, MedRxiv 23300495.
- Sarusie MVK, Rönnbäck C, Jespersgaard C, Baungaard S, Ali Y, Christensen ST, Brøndum-Nielsen K, Møllgård K, Rosenberg T, Larsen LA, Grønskov K (2024) A novel GFAP frameshift variant identified in a family with optico-retinal dysplasia and vision impairment. Hum Mol Genet 33:2145-2158.
- Audain E, Wilsdon A, Breckpot J, Izarzugaza JMG, Fitzgerald TW, Kahlert AK, Sifrim A, Wünnemann F, Perez-Riverol Y, Abdul-Khaliq H, Bak M, Bassett AS, Belmont JW, Benson DW, Berger F, Daehnert I, Devriendt K, Dittrich S, Daubeney P, Garg V, Hackmann K, Hoff K, Hofmann P, Dombrowsky G, Pickardt T, Bauer U, Keavney BD, Klaassen S, Kramer HH, Marshall CR, Milewicz DM, Lemaire SA, Coselli J, Mitchell ME, Tomita-Mitchell A, Prakash SK, Stamm K, Stewart AFR, Silversides CK, Siebert R, Stiller B, Rosenfeld JA, Vater I, Postma AV, Caliebe A, Brook JD, Andelfinger G, Hurles ME, Thienpont B, Larsen LA, Hitz MP (2021) Integrative analysis of genomic variants reveals new associations of candidate haploinsufficient genes with congenital heart disease. PLoS Genet 17:e1009679
- Farooq M, Lindbæk L, Krogh N, Doganli C, Keller C, Mönnich M, Sakthivel S, Mang Y, Fatima A, Andersen VS, Hussain MS, Eiberg H, Hansen L, Kjaer KW, Gopalakrishnan J, Pedersen LB, Møllgård K, Nielsen H, Baig SM, Tommerup N, Christensen ST and Larsen LA (2020) RRP7A links primary microcephaly to dysfunction of ribosome biogenesis, resorption of primary cilia and neurogenesis. Nat Commun 11:5816.
- Izarzugaza JMG, Ellesøe SG, Doganli C, Ehlers NS, Dalgaard MD, Audain E, Dombrowsky G, Banasik K, Sifrim A, Wilsdon A, Thienpont B, Breckpot J, Gewillig M, Competence Network for Congenital Heart Defects Germany, Brook JD, Hitz MP, Larsen LA, Brunak S (2020) Systems genetics analysis identifies calcium signaling defects as novel cause of congenital heart disease. Genome Med 12:76.
- Schroeder AM, Allahyari M, Vogler G, Missinato MA, Nielsen T, Yu MS, Theis JL, Larsen LA, Goyal P, Rosenfeld J, Nelson TJ, Olson TM, Colas AR, Grossfeld P, Bodmer R (2019) Model System Identification of Novel Congenital Heart Disease Gene Candidates: focus on RPL13. Hum Mol Genet 28:3954-3969.
- Ellesøe SG, Workman CT, Bouvagnet P, Loffredo CA, McBride KL, Hinton RB, van Engelen K, Gertsen EC, Mulder BJM, Postma AV, Anderson RH, Hjortdal VE, Brunak S, Larsen LA (2018) Familial co-occurrence of congenital heart defects follows distinct patterns. Eur Heart J 39:1015-22.
- Mönnich M, Borgeskov L, Breslin L, Jakobsen L, Rogowski M, Doganli C, Schrøder JM, Mogensen JB, Blinkenkjær L, Harder LM, Lundberg E, Geimer S, Christensen ST, Andersen JS, Larsen LA, Pedersen LB (2018) CEP128 localizes to the subdistal appendages of the mother centriole and regulates TGF-β/BMP signaling at the primary cilium. Cell reports 22:2584-2592.
- Schmid F, Schou KB, Vilhelm MJ, Holm MS, Breslin L, Farinelli P, Larsen LA, Andersen JS, Pedersen LB, Christensen ST (2018) IFT20 modulates ciliary PDGFRα signaling by regulating the stability of Cbl E3 ubiquitin ligases. J Cell Biology 217:151-161.
- Andersen TA, Troelsen KDL, Larsen LA (2014) Of Mice and Men: Molecular Genetics of Congenital Heart Disease. Cell Mol Life Sci 71:1327-52
- Clement CA, Ajbro KD, Koefoed K, Vestergaard ML, Veland IR, Henriques de Jesus MP, Pedersen LB, Benmerah A, Andersen CY, Larsen LA, Christensen ST (2013) TGF-β Signaling Is Associated with Endocytosis at the Pocket Region of the Primary Cilium. Cell Reports 3:1806-14.
- Lage K, Greenway S, Rosenfeld JA, Wakimoto H, Gorham JM, Segre A, Roberts AE, Smoot LB, Pu WT, Pereira AC, Mesquita SM, Tommerup N, Brunak S, Ballif BC, Schaffer L, Donahoe PK, Daly MJ, Seidman JG, Seidman CE and Larsen LA (2012) Genetic and environmental risk factors in congenital heart disease functionally converge in protein networks driving heart development. Proc Natl Acad Sci U S A 109:14035-40.
- Thienpont B, Zhang L, Postma AV, Breckpot J, Tranchevent L-C, Van Loo P, Møllgård K, Tommerup N, Bache I, Tümer Z, van Engelen K, Menten B, Mortier G, Waggoner D, Gewillig M, Moreau Y, Devriendt K and Larsen LA (2010) Haplo-insufficiency of TAB2 causes congenital heart defects in humans. Am J Hum Genet 86:1-11.
- Lage K, Møllgård K, Greenway S, Wakimoto H, Gorham JM, Workman CT, Bendsen E, Hansen NT, Rigina O, Roque FS, Wiese C, Christoffels VM, Roberts AE, Smoot LB, Pu WT, Donahoe PK, Tommerup N, Brunak S, Seidman CE, Seidman JG and Larsen LA (2010) Dissecting spatio-temporal protein networks driving human heart development and related disorders. Mol Syst Biol 6:381.
- Erdogan F, Larsen LA, Zhang L, Tümer Z, Tommerup N, Chen W, Jacobsen JR, Schubert M, Jurkatis J, Tzschach A, Ropers H-H and Ullmann R. (2008) High frequency of submicroscopic genomic aberrations detected by tiling path array CGH in patients with isolated congenital heart disease. J Med Genet 45:704-709.
We express our sincere gratitude for the generous support we have received over the years from:
- Aase og Einar Danielsen's Fond
- A.P. Møller Foundation
- Arvid Nilssons Fond
- Brødrene Hartmanns Fond
- Carlsberg Foundation
- Dagmar Marschall's fond
- Danish Cardiovascular Academy
- Danish Childrens Heart Foundation
- Danish Heart Foundation
- Danish National Research Foundation
- Direktør Jacob Madsens og Hustru Olga Madsens Fond
- European Union
- Helge Peetz og Verner Peetz og hustru Vilma Peetz Legat
- Independent Research Fund Denmark
- Kirsten & Freddy Johansens Fond
- Lundbeck Foundation
- Læge Sophus Carl Emil Friis og hustru Olga Doris Friis ́ Legat
- Novo Nordisk Foundation
- Oda og Hans Svenningsens Fond
- Ronald McDonals House Charities
- UCPH excellence program for interdisciplinary research
 Group Leader
Group Leader
Lars Allan Larsen
Professor of Developmental Genetics
larsal@sund.ku.dk
(+45) 35 32 78 27
CV, publications, etc



