- Department of Cellular and Molecular Medicine
- Research Groups
- Messerschmidt Group
Messerschmidt Group
Our laboratory investigates the role of epigenetic and transcriptional regulators creating and maintaining a conducive environment for mammalian germ cell formation, oocyte-to-embryo transition and early embryogenesis.
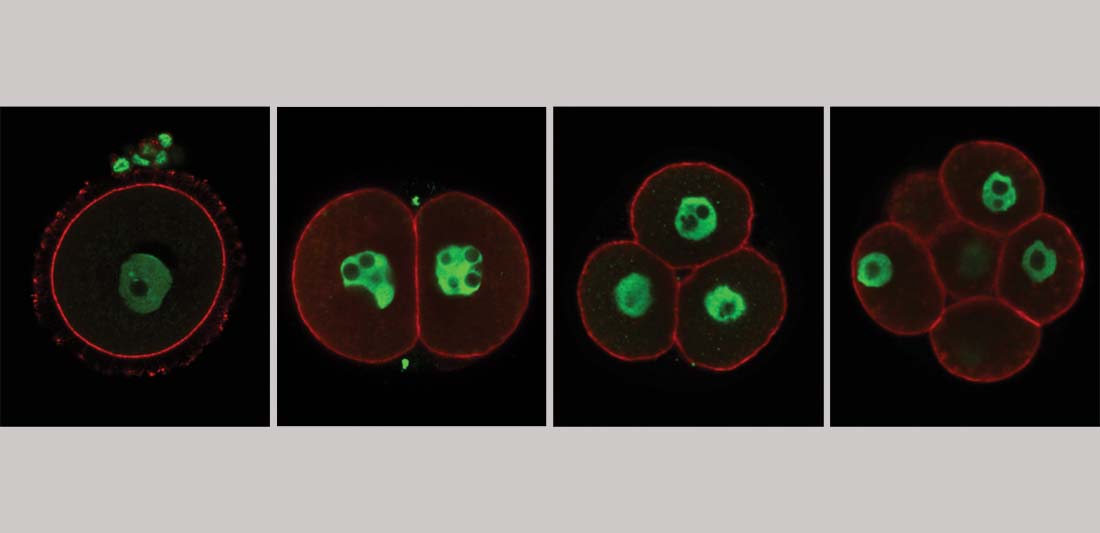
Mammalian Developmental Epigenetics
The inheritance of epigenetic features across generations and their impact on development and health is an enigmatic aspect in mammalian epigenetic research. In lay man’s terms, development and well-being of humans is not only defined by the information encoded in our DNA, but also by how this information is used. This is driven by chemical (epigenetic) modifications allowing or restricting access to the genetic information without altering the DNA code itself. Defects in this epigenome can be equally damaging to health and development as mutations to the DNA itself. The genome of newly formed embryo is stripped of most epigenetic information, yet, an unknown portion of the parental epigenome is transmitted with the genetic material at fertilization and fulfils important roles in the embryonic development and health of the progeny. Revealing the heritable epigenetic information, its functional relevance and the machineries involved in the transmission in humans and mice is the essence of our work.
Our laboratory investigates the role of epigenetic and transcriptional regulators creating and maintaining a conducive environment for mammalian germ cell formation, oocyte-to-embryo transition and early embryogenesis. Our main focus is on maternal factors conveying functional epigenetic inheritance from germline to soma or even across multiple generations. It’s our aim to expose molecular components, mechanisms and targets, which when defective, contribute to reproductive or developmental disease or disrupted homeostasis. We employ in vitro and in vivo models ranging from pluripotent stem cells to powerful genetic mouse models and human models in combination with state of the art next-generation sequencing approaches to shed light into the complex epigenetic and transcriptional dynamics at this crucial aspect of mammalian development.
- Han B, Seah MKY, Brooks I, Quek D, Huxley H, Foo CS, Lee LT, Wollmann H, Guo H, Messerschmidt DM#, Guccione E#. PRDM10 regulates early embryogenesis and embryonic stem cell homeostasis through maintenance of global translation (IF 10). Nat Commun. 2020 Jul 17;11(1):3603. doi: 10.1038/s41467-020-17304-3 (#corresponding authors)
- Tan JHL, Wollmann H, van Pelt AMM, Kaldis P, Messerschmidt DM. Infertility-causing haploinsufficiency reveals TRIM28/KAP1 requirement in spermatogonia (IF 5.4). Stem Cell Reports. 2020 May 12;14(5):818-827. doi: 10.1016/j.stemcr.2020.03.013
- Seah MKY, Wang Y, Goy PV, Loh HM, Peh WJ, Loh DHP, Han BY, Wong E, Leong EL, Wolf G, Mzoughi S, Wollmann H, Macfarlan TS, Guccione E, Messerschmidt DM. The KRAB-Zinc finger protein ZFP708 mediates epigenetic repression at RMER19B retrotransposons. (IF 5.7 2018). Development. 2019 Mar 7. doi: 10.1242/dev.170266.
- Mzoughi S, Zhang J, Hequet D, Teo SX, Fang H, Xing QR, Bezzi M, Seah MKY, Ong SLM, Shin EM, Wollmann H, Wong ESM, Al-Haddawi M, Stewart CL, Tergaonkar V, Loh YH, Dunn NR, Messerschmidt DM, Guccione E. PRDM15 safeguards naive pluripotency by transcriptionally regulating WNT and MAPK-ERK signaling. (IF 27.1 2017). Nat Genet. 2017 Jul 24. doi: 10.1038/ng.3922.
- Sampath Kumar A, Seah MK, Ling KY, Wang Y, Tan JH, Nitsch S, Lim SL, Lorthongpanich C, Wollmann H, Low DH, Guccione E, Messerschmidt DM. Loss of maternal Trim28 causes male-predominant early embryonic lethality. (IF 9.4). Genes Dev. 2017 Jan 1;31(1):12-17. doi: 10.1101/gad.291195.116.
- Cheow LF, Quake SR, Burkholder WF, Messerschmidt DM. Multiplexed locus-specific analysis of DNA methylation in single cells. (IF 9.6). Nat Protoc. 2015 Apr;10(4):619-31. doi: 10.1038/nprot.2015.041.
- Lorthongpanich C, Cheow LF, Balu S, Quake SR, Knowles BB, Burkholder WF, Solter D, Messerschmidt DM. Single-cell DNA-methylation analysis reveals epigenetic chimerism in preimplantation embryos. (IF 31.4). Science. 2013 Sep 6;341(6150):1110-2. doi: 10.1126/science.1240617.
- Messerschmidt DM#, de Vries W, Ito M, Solter D, Ferguson-Smith A, Knowles BB. Trim28 is required for epigenetic stability during mouse oocyte to embryo transition. (IF 31). Science. 2012 Mar 23;335(6075):1499-502. doi: 10.1126/science.1216154. (#corresponding author)
We welcome applications from motivated postdoctoral and predoctoral researchers as well as master students. Please contact us at danielm@sund.ku.dk
Group Leader
 Daniel Messerschmidt
Daniel Messerschmidt
associate professor
NNF Young Investigator
danielm@sund.ku.dk
(+45) 35 32 02 86
CV, Publications, etc

