Vakhrushev Group
The research focus of Vakhrushev Group is proteome-wide characterization of O-glycan structures at specific sites in O-glycoproteins.
Post-translational modifications (PTMs) provide for a nearly limitless expansion of the structural space of the proteome of an organism and add manifold layers of complexity to the interactome. Protein glycosylation is arguably the most abundant and certainly the most diverse PTM. Of the two major classes of glycosylation - those occurring at Asn residues (N-linked) and those occurring at Ser, Thr, or Tyr residues (O-linked) - mucin-type (GalNAc-type) O-glycosylation is the most diverse and differentially regulated and yet the least understood. Site-specific O-glycosylation is emerging as an important concept for regulating protein processing and functions. However, full understanding of the nature and functions of this abundant type of protein glycosylation is severely hampered by lack of tools and methods for proteome-wide characterization of O-glycan structures at specific sites in O-glycoproteins.
In the field of O-glycoproteomics the analysis of glycan structures is already rather well established while the localization and quantification of O-glycans on single sites of proteins still offers considerable challenges. We have recently made a significant breakthrough in proteome-wide discovery of localization of O-glycosites (O-GalNAc, O-Man) by developing a state-of-the-art strategy based on mass spectrometry applied to genetically engineered cells, so-called “SimpleCells”, which express reduced glycan complexity. Using such modified cells a general answer to the problem of identification of O-glycosylation in proteins and determination of O-glycans attached in a global high-throughput manner was provided, e.g. reporting >15000 O-glycosites and >5000 O-glycoproteins.
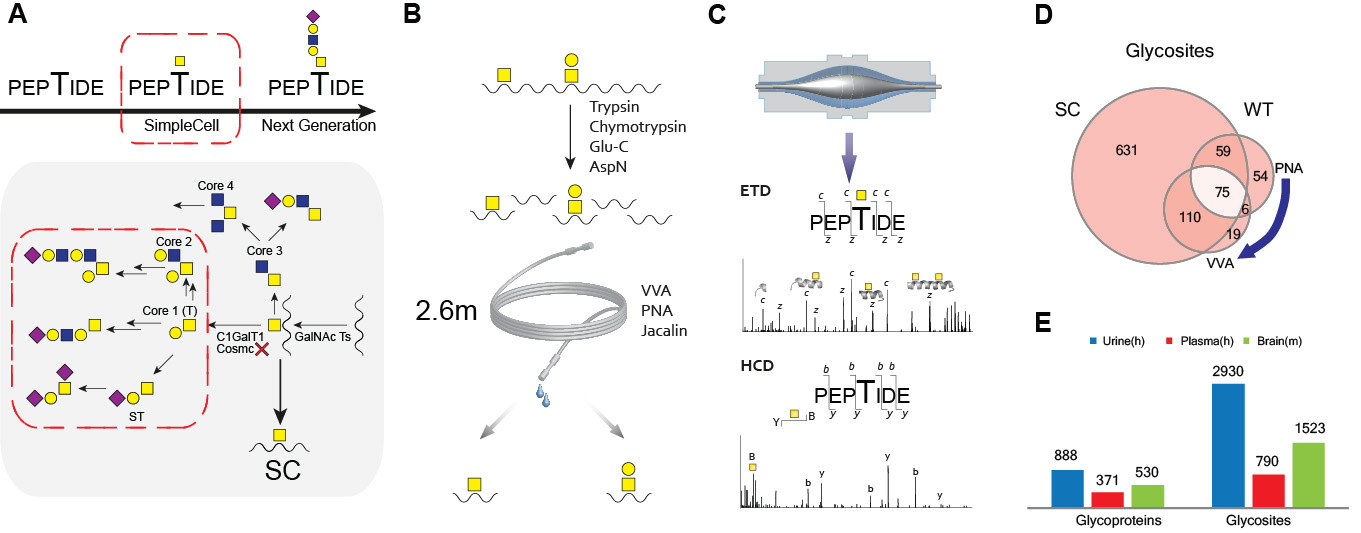
A) Schematic depiction of SimpleCell strategy. COSMC POMGNT1 knockout results in simplified O-GalNAc or NeuAcα2-6GalNAc (in some cells) glycosylations. B) Lectin weak affinity chromatography (LWAC) enrichment strategy of O-GalNAc glycopeptides by Vicia villosa agglutinin (VVA) and Gal(b1-3)GalNAc glycopeptides by Peanut agglutinin (PNA) or Jacalin. C) Outline of the glycoproteomics workflow. O-GalNAc glycopeptides were submitted to nLC–MS (OrbiTrap) followed by repetitive top 5 HCD MS2 and top 5 ETD MS2 analysis. D) Analysis of the CHO WT O-glycoproteome. Comparative analysis of a tryptic digest subset of O-glycosites identified in CHO SC (left) and a tryptic digest of CHO WT (right). E)An example of O-glycoproteome analysis of biofluids (human urine and plasma) and tissue samples (mouse brain).
Next Generation Glycoproteomics
The major obstacle is around two fundamental problems: 1) to overcome the difficulty of simultaneous identification and quantification of native glycan structures on single glycosites and 2) address glycosite occupancy and stoichiometry especially in complex matrix.
The Next Generation Glycoproteomics workflow based on the SimpleCell glycoproteome strategies to tackle the challenges of developing what will be the next breakthrough in PTM proteomics, namely high throughput glycoproteomics. This
approach should allow screening of complex samples under simultaneous identification of native glyco structures and glycosites. To advance quantitative glycoproteomics strategy development of high-throughput protocols for determination of glycosylation site occupancy will represent a next step. We will be able to simultaneously report site localization, and at the same time determine glycosylation stoichiometry and explore glycosylation dynamics. Wider application of these strategies should expand our knowledge of the different glycoproteomes and open for discovery of biological functions in health and disease. The strategy should thus have substantial impact for the cell biology field and even more widely in biomedicine. Moreover, the proposed strategy is likely to be highly applicable for other types of protein glycosylation and more generally to other complex posttranslational modifications.
Tyrosine O-GalNAc glycosylation
Along with expansion of the O-glycoproteome an interesting new development was the discovery of GalNAc attachment to tyrosine residues. We have currently identified a total of more than 150 Tyr O-glycosites. Currently, our understanding of the biology of this new modification of extracellular proteins is limited, and a first step is to further define this subset of the O-GalNAc glycoproteome. However, recently, phosphorylation of Tyr residues on membrane proteins have been identified on many of the same proteins in a close proximity where we have identified GalNAc-Tyr glycosites (Bordoli M.L. et al. Cell 2014). The aim is to develop a protocol for a selective enrichment of GalNAc-Tyr O-glycopeptides and further expand this O-glycoproteome. It is not yet known if the polypeptide GalNAc-T enzymes control this glycosylation or other unknown enzymes, but a potential for cross-talk between phosphorylation and GalNAc-type glycosylation on tyrosine residues can be proposed.
Clinical Glycoproteomics
Aberrant glycosylation occurs during cancer development. In O-glycosylation it leads into truncation of O-glycostructures towards NeuAc-GalNAc structure (SiaTn-epitope). As the result up regulation of SiaTn-glycopeptides could serve as a source of potential biomarkers. According to the project design next generation glycoproteomics platform is to be extended and applied for investigation of aberrant glycosylation in human bio fluids (plasma, urine) and tissues (liver, kidney, brain) of patient and as a groundbreaking tool in clinical studies.
Computational Glycoproteomics and GlycoInformatics
Modern proteomics and mass spectrometry requires an integration of strong bioinformatics and programming. Glycoproteomics area is still lacking of robust informatics tools especially for high throughput applications. The aim is to further develop and improve algorithms for mass spectrometry based glycoproteomics.
- Steentoft, C, Vakhrushev, SY, Bennett, EP., Vester-Christensen, MB., Schjoldager, KB., Kong, Y, Mandel, U, Wandall, HH., Levery, S, and Clausen, H. Mining the O-glycoproteome using zinc-finger nuclease-glycoengineered SimpleCell lines. Nat. Methods, 2011, 8(11):977-982
- Steentoft C, Vakhrushev SY, Joshi HJ, Kong Y, Vester-Christensen MB, Schjoldager KTG, Lavrsen K, Dabelsteen S, Pedersen NB, Marcos-Silva L, Gupta R, Bennett EP, Mandel U, Wandall H, Brunak S, Levery SB, Clausen H (2013) Precision Mapping of the Human GalNAc-Type O-Glycoproteome – First Generation View From 12 Human SimpleCells. EMBO J 32(10):1478-88
- Vester-Christensen MB, Halim A, Joshi HJ, Steentoft C, Bennett EP, Levery SB, Vakhrushev SY, Clausen H (2013) Mining the O-mannose glycoproteome reveals cadherins as major O-mannosylated glycoproteins. Proc Natl Acad Sci USA 110(52):21018-23
- Levery SB, Steentoft C, Halim AH, Narimatsu Y, Clausen H, Vakhrushev SY. Advances in Mass Spectrometry Driven O-Glycoproteomics Biochim Biophys Acta. 2015, 1850(1): 33-42.
- Yang Z, Wang S, Halim A, Schulz MA, Frodin M, Rahman SH, Vester-Christensen MB, Behrens C, Kristensen C, Vakhrushev SY, Bennett EP, Wandall HH, Clausen H. (2015) Engineered CHO cells for production of diverse, homogeneous glycoproteins. Nat Biotechnol 33(8):842-4
- Campos D, Freitas D, Gomes J, Magalhães A, Steentoft C, Gomes C, Vester-Christensen MB, Ferreira JA, Afonso LP, Santos LL, de Sousa JP, Mandel U, Clausen H, Vakhrushev SY, Reis CA. Probing the O-glycoproteome of Gastric Cell Lines for Biomarker Discovery. Mol. Cell Proteom. 2015, 14(6): 1616-29
- Khetarpal SA, Schjoldager KTG, Christoffersen C, Raghavan A, Edmondson AC, Reutter HM, Ahmed B, Ouzzani R, Peloso GM, Vitali C, Zhao W, Varshini A, Millar JS, Park Y, Fernando G, Livanov V, Choi S, Noé E, Patel P, Ho S, Global Lipids Genetics Consortium, Kirchgessner TG, Wandall HH, Hansen L, Bennett EP, Vakhrushev SY, Saleheen D, Kathiresan S, Brown CD, Jamra AB, LeGuern E, Clausen H, Rader DJ. (2016) Loss of function of GALNT2 lowers high density lipoproteins in humans, nonhuman primates, and rodents. Cell Metabolism 24(2):234-45.
- Larsen ISB, Narimatsu Y, Joshi HJ, Yang Z, Harrison OJ, Brasch J, Shapiro L, Honig B, Vakhrushev SY, Clausen H, Halim A. (2017) Mammalian O-mannosylation of Cadherins and Plexins is Independent of Protein O-mannosyltransferase 1 and 2. J Biol Chem 292(27):11586-11598
- Larsen ISB, Narimatsu Y, Joshi HJ, Siustaite L, Harrison OJ, Brasch J, Goodman K, Hansen L, Shapiro L, Honig B, Vakhrushev SY, Clausen H, Halim A. (2017) Discovery of a novel O-mannosylation pathway selectively serving cadherins and protocadherins. Proc Natl Acad Sci USA 114(42):11163-11168
- Ramon Hurtado-Guerrero, Zaragoza
- Leonor David, Porto
- Celso Reis, Porto
- Fred Bard, Singapore
- Tim Rozhdestvenski, Muenster
- Peter Brodersen, Copenhagen
- Janosh Terzic, Split
- Andrea Urbani, Rome
- Karl Mechtler, Wien
- Lance Wells, Athens, US
- Danish National Research Foundation
- Lundbeck Foundation
- AP Møller
- NIH
- EU
 Group Leader
Group Leader
Sergey Vakhrushev
Associate Professor
seva@sund.ku.dk
(+45) 35 32 77 85 / (+45) 23 84 01 54
CV, publications, etc
