Vogel Group
The Vogel laboratory explores the interplay between the serine proteases matriptase and prostasin, and the inhibitors/ally proteins HAI-1 and HAI-2, to design new treatment(s) for cancer patients.
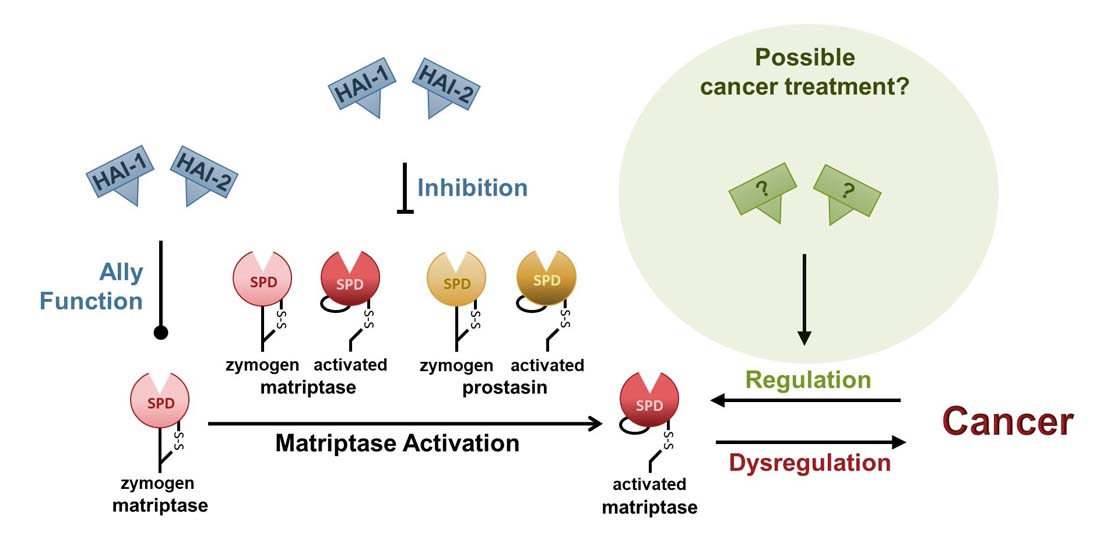
Is a cure for cancer within reach?
Research in mice has shown that over-expression of the transmembrane serine protease, matriptase, single-handedly causes tumors with high efficiency [1]. Matriptase is located on the basolateral plasma membrane of all epithelial cells [2, 3] and all epithelial cell-derived cancers [4], and catalyzes the conversion of signal molecules, leading to the activation of signal transduction pathways [5, 6]. Under normal conditions, matriptase is kept under strict control by the serine protease inhibitors HAI-1 and HAI-2 [7, 8]. Up to 90% of cancers are believed to be of epithelial origin, and most display dysregulated expression of matriptase, HAIs, or both [4]. Indicating that the matriptase regulatory system is involved in most epithelial-derived cancers.
Mouse model studies have shown that high expression of matriptase leads to malignant cancer that can be prevented by simultaneous over-expression of either HAI-1 or HAI-2 [1, 9]. In 2015 it was shown that over-expression of HAI-2 caused regression and remission of individual, established, and epithelial-derived malignant tumors in a mouse model [9]. Similar results have also been obtained using human prostate cancer cells in a mouse model [10]. These studies suggest that sufficient levels of HAIs can reverse carcinogenesis and treat epithelial-derived malignant cancers.
It appears that activation of matriptase is necessary for the development of cancer, as over-expression of a matriptase protein that can be activated, leads to cancer [1], whereas over-expression of zymogen-locked matriptase does not [11]. Matriptase activation can be auto-catalyzed by the active zymogen form of another matriptase or by another activated matriptase [12]. The protease can also be activated by the active zymogen form of the serine protease prostasin, or by activated prostasin [13]. All four activators can be inhibited by HAI-1 and HAI-2.
In addition to being serine protease inhibitors, HAI-1 and HAI-2 are also ally proteins, acting on the newly synthesized zymogen matriptase, preventing its post-translational degradation by an unknown mechanism [14-16]. At present, it is unclear whether it is the inhibitory function of the HAIs, their ally function, or both combined that can cause carcinogenesis to reverse and tumors to regress.
The goal of the Vogel laboratory is to provide a basic understanding of the interplay between matriptase, prostasin, HAI-1, and HAI-2 to design treatment(s) for cancer and other diseases.
References
- List K, Szabo R, Molinolo A, Sriuranpong V, Redeye V, Murdock T, et al. Deregulated matriptase causes ras-independent multistage carcinogenesis and promotes ras-mediated malignant transformation. Genes Dev. 2005;19(16):1934-50.
- List K, Szabo R, Molinolo A, Nielsen BS, Bugge TH. Delineation of matriptase protein expression by enzymatic gene trapping suggests diverging roles in barrier function, hair formation, and squamous cell carcinogenesis. Am J Pathol. 2006;168(5):1513-25.
- Friis S, Godiksen S, Bornholdt J, Selzer-Plon J, Rasmussen HB, Bugge TH, et al. Transport via the transcytotic pathway makes prostasin available as a substrate for matriptase. J Biol Chem. 2011;286(7):5793-802.
- List K. Matriptase: a culprit in cancer? Future Oncol. 2009;5(1):97-104.
- Szabo R, Rasmussen AL, Moyer AB, Kosa P, Schafer JM, Molinolo AA, et al. c-Met-induced epithelial carcinogenesis is initiated by the serine protease matriptase. Oncogene. 2011;30(17):2003-16.
- Sales KU, Friis S, Konkel JE, Godiksen S, Hatakeyama M, Hansen KK, et al. Non-hematopoietic PAR-2 is essential for matriptase-driven pre-malignant progression and potentiation of ras-mediated squamous cell carcinogenesis. Oncogene. 2015;34(3):346-56.
- Szabo R, Molinolo A, List K, Bugge TH. Matriptase inhibition by hepatocyte growth factor activator inhibitor-1 is essential for placental development. Oncogene. 2007;26(11):1546-56.
- Szabo R, Hobson JP, Christoph K, Kosa P, List K, Bugge TH. Regulation of cell surface protease matriptase by HAI2 is essential for placental development, neural tube closure and embryonic survival in mice. Development. 2009;136(15):2653-63.
- Sales KU, Friis S, Abusleme L, Moutsopoulos NM, Bugge TH. Matriptase promotes inflammatory cell accumulation and progression of established epidermal tumors. Oncogene. 2015;34(35):4664-72.
- Tsai CH, Teng CH, Tu YT, Cheng TS, Wu SR, Ko CJ, et al. HAI-2 suppresses the invasive growth and metastasis of prostate cancer through regulation of matriptase. Oncogene. 2014;33(38):4643-52.
- Friis S, Tadeo D, Le-Gall SM, Jürgensen HJ, Sales KU, Camerer E, et al. Matriptase zymogen supports epithelial development, homeostasis and regeneration. BMC Biol. 2017;15(1):46.
- Skovbjerg S, Holt-Danborg L, Nonboe AW, Hong Z, Frost Á K, Schar CR, et al. Inhibition of an active zymogen protease: the zymogen form of matriptase is regulated by HAI-1 and HAI-2. The Biochemical journal. 2020;477(9):1779-94.
- Holt-Danborg L, Skovbjerg S, Goderum KW, Nonboe AW, Stankevic E, Frost Á K, et al. Insights into the regulation of the matriptase-prostasin proteolytic system. The Biochemical journal. 2020;477(22):4349-65.
- Nonboe AW, Bald ZH, Vogel LK. Understanding HAIs: Ally proteins in the fight against cancer. Febs j. 2022;289(12):3416-8.
- Oberst MD, Chen LY, Kiyomiya K, Williams CA, Lee MS, Johnson MD, et al. HAI-1 regulates activation and expression of matriptase, a membrane-bound serine protease. Am J Physiol Cell Physiol. 2005;289(2):C462-70.
- Nonboe AW, Krigslund O, Soendergaard C, Skovbjerg S, Friis S, Andersen MN, et al. HAI-2 stabilizes, inhibits and regulates SEA-cleavage-dependent secretory transport of matriptase. Traffic. 2017;18(6):378-91.
A treatment for established malignant tumors
Mouse model studies have shown that high expression of Matriptase leads to malignant cancer and that this can be prevented by simultaneous over-expression of either HAI-1 or HAI-2 [1, 2]. Similar results have also been obtained using human prostate cancer cells in a mouse model [3]. These studies suggest that sufficient levels of HAIs can reverse carcinogenesis and treat epithelial-derived malignant cancers. Unfortunately, HAIs are membrane-bound proteins and cannot be used clinically.
Inhibitors of the Matriptase-activators are likely to be found within the human genome, as they are still a highly understudied group of proteins and serine protease inhibitors generally display high promiscuity, which is reflected in the HAI’s ability to inhibit all four Matriptase-activators in addition to several other known serine proteases.
Our laboratory has, as the only one in the world, established several assays detecting inhibition of the activators of matriptase, allowing us to screen for inhibitors better suited for clinical use.
The HAIs unique ally protein function may also be involved in reversing carcinogenesis. Therefore, we simultaneously investigate the HAIs ally protein mechanism.
Epithelial-derived cancers are sometimes detected late or located in places difficult to reach with conventional treatments, like surgery and chemotherapy. Cancer therapy based on a systemic application of a safe protease inhibitor will therefore be a huge advantage and could save many lives.
Matriptase variants cause autosomal, recessive, congenital, ichthyosis 11
Ichthyosis is a common disease that causes "fish-scale" like skin with poor treatment options. The molecular mechanisms behind ichthyosis are not understood, but variants in several genes, including the filaggrin gene, cause ichthyosis.
Matriptase knock-out mice [4] and prostasin knock-out mice [5] both lack profilaggrin processing, which has also been observed in a patient with ichthyosis caused by a matriptase variant [6]. This strongly suggests that matriptase, prostasin, and filaggrin are part of the same enzymatic cascade, which causes ichthyosis when its function is abrogated.
Multiple variants in the ST14 gene encoding the serine protease matriptase lead to a type of ichthyosis called Autosomal, Recessive, Congenital, Ichthyosis 11 (ARCI11). We are currently investigating the proteolytic properties of the ARCI11-causing matriptase variants.
The frequency of ARCI11 is elusive at present. We have genetic material available to do a systematic search for other ARCI11-causing variants of matriptase. We plan to screen this material for new variants and estimate the frequency of both new and known ARCI11 variants. We believe that information about the matriptase variants will lead to a better understanding of ichthyosis and improve treatment options.
References
- Sales, K.U., et al., Matriptase promotes inflammatory cell accumulation and progression of established epidermal tumors. Oncogene, 2015. 34(35): p. 4664-72.
- List, K., et al., Deregulated matriptase causes ras-independent multistage carcinogenesis and promotes ras-mediated malignant transformation. Genes Dev, 2005. 19(16): p. 1934-50.
- Tsai, C.H., et al., HAI-2 suppresses the invasive growth and metastasis of prostate cancer through regulation of matriptase. Oncogene, 2014. 33(38): p. 4643-52.
- List, K., et al., Loss of proteolytically processed filaggrin caused by epidermal deletion of matriptase/MT-SP1. Journal of Cell Biology, 2003. 163(4): p. 901-910.
- Leyvraz, C., et al., The epidermal barrier function is dependent on the serine protease CAP1/Prss8. Journal of Cell Biology, 2005. 170(3): p. 487-496.
- Alef T, Torres S, Hausser I, Metze D, Türsen U, Lestringant GG, Hennies HC. Ichthyosis, follicular atrophoderma, and hypotrichosis caused by mutations in ST14 is associated with impaired profilaggrin processing. J Invest Dermatol. 2009 Apr;129(4):862-9. doi: 10.1038/jid.2008.311.
Holt-Danborg L, Skovbjerg S, Goderum KW, Nonboe AW, Stankevic E, Frost ÁK, Vitved L, Jensen JK, Vogel LK. Insights into the regulation of the matriptase-prostasin proteolytic system. Biochem J. 2020 Nov 27;477(22):4349-4365. doi: 10.1042/BCJ20200630.
Skovbjerg S, Holt-Danborg L, Nonboe AW, Hong Z, Frost ÁK, Schar CR, Thomas CC, Vitved L, Jensen JK, Vogel LK. Inhibition of an active zymogen protease: the zymogen form of matriptase is regulated by HAI-1 and HAI-2. Biochem J. 2020 May 15;477(9):1779-1794. doi: 10.1042/BCJ20200182.
Holt-Danborg L, Vodopiutz J, Nonboe AW, De Laffolie J, Skovbjerg S, Wolters VM, Müller T, Hetzer B, Querfurt A, Zimmer KP, Jensen JK, Entenmann A, Heinz-Erian P, Vogel LK, Janecke AR. SPINT2 (HAI-2) missense variants identified in congenital sodium diarrhea/tufting enteropathy affect the ability of HAI-2 to inhibit prostasin but not matriptase. Hum Mol Genet. 2019 Mar 1;28(5):828-841. doi: 10.1093/hmg/ddy394.
Sales KU, Friis S, Konkel JE, Godiksen S, Hatakeyama M, Hansen KK, Rogatto SR, Szabo R, Vogel LK, Chen W, Gutkind JS, Bugge TH. Non-hematopoietic PAR-2 is essential for matriptase-driven pre-malignant progression and potentiation of ras-mediated squamous cell carcinogenesis. Oncogene. 2015 Jan 15;34(3):346-56. doi: 10.1038/onc.2013.563. Epub 2014 Jan 27.
Tamberg T, Hong Z, De Schepper D, Skovbjerg S, Dupont DM, Vitved L, Schar CR, Skjoedt K, Vogel LK, Jensen JK. Blocking the proteolytic activity of zymogen matriptase with antibody-based inhibitors. J Biol Chem. 2019 Jan 4;294(1):314-326. doi: 10.1074/jbc.RA118.004126. Epub 2018 Nov 8.
Nonboe AW, Krigslund O, Soendergaard C, Skovbjerg S, Friis S, Andersen MN, Ellis V, Kawaguchi M, Kataoka H, Bugge TH, Vogel LK. HAI-2 stabilizes, inhibits and regulates SEA-cleavage-dependent secretory transport of matriptase. Traffic. 2017 Jun;18(6):378-391. doi: 10.1111/tra.12482. Epub 2017 May 2.
Friis S, Uzzun Sales K, Godiksen S, Peters DE, Lin CY, Vogel LK, Bugge TH. A matriptase-prostasin reciprocal zymogen activation complex with unique features: prostasin as a non-enzymatic co-factor for matriptase activation. J Biol Chem. 2013 Jun 28;288(26):19028-39. doi: 10.1074/jbc.M113.469932. Epub 2013 May 14.
Szabo R, Uzzun Sales K, Kosa P, Shylo NA, Godiksen S, Hansen KK, Friis S, Gutkind JS, Vogel LK, Hummler E, Camerer E, Bugge TH. Reduced prostasin (CAP1/PRSS8) activity eliminates HAI-1 and HAI-2 deficiency-associated developmental defects by preventing matriptase activation. PLoS Genet. 2012;8(8):e1002937. doi: 10.1371/journal.pgen.1002937. Epub 2012 Aug 30.
Friis S, Godiksen S, Bornholdt J, Selzer-Plon J, Rasmussen HB, Bugge TH, Lin CY, Vogel LK. Transport via the transcytotic pathway makes prostasin available as a substrate for matriptase. J Biol Chem. 2011 Feb 18;286(7):5793-802. doi: 10.1074/jbc.M110.186874. Epub 2010 Dec 10.
Delmas B, Gelfi J, L'Haridon R, Vogel LK, Sjöström H, Norén O, Laude H. Aminopeptidase N is a major receptor for the entero-pathogenic coronavirus TGEV. Nature. 1992 Jun 4;357(6377):417-20. doi: 10.1038/357417a0.
- Adam Frederik Sander Pedersen, University of Copenhagen, Copenhagen, Denmark
- Chen-Yong Lin, University of Maryland, Maryland, USA
- Christine Schar, University of Aarhus, Aarhus, Denmark
- Hans Eiberg, University of Copenhagen, Copenhagen, Denmark
- Hiroaki Kataoka, University of Miyazaki, Miyazaki, Japan
- Jan K. Jensen, University of Aarhus, Aarhus, Denmark
- Lars Ellgaard, University of Copenhagen, Copenhagen, Denmark
- Makiko Kawaguchi, University of Miyazaki, Miyazaki, Japan
- Roman Szabo, NIH, Bethesda, Maryland, USA
- Thomas H. Bugge, NIH, Bethesda, Maryland, USA
We currently have available positions for BSc and MSc students interested in doing a project or thesis on matriptase in cancer, prostasin in ichthyosis, or a related topic. If you want to know more about being a student in the Vogel lab, our previous student Klaudia Parsberg Jensen, MSc Molecular Biomedicine, made a website about her experience with the lab: matriptase.com
If you are interested in doing a project, please contact; vogel@sund.ku.dk and include a CV, your most recent transcripts, and a statement about your project and area of interest.

Desirée og Niels Ydes Fond
![]()
Den lægevidenskabelige del af Det Sundhedsvidenskabelige Fakultets fond for videnskabeligt ansatte kandidater og studerende ved Københavns Universitet
Group Leader
 Lotte Vogel
Lotte Vogel
associate professor
vogel@sund.ku.dk
(+45) 35 33 55 52 / (+45) 30 56 43 96
CV, Publications, etc
